Diamonds are Forever: Using NIR To Find the Fakes
Researchers used spectroscopy and time-gated near-infrared luminescence to reveal cubic zirconia in batches of tiny diamonds
In the mid-1950s, Ian Fleming met with Sir Percy Sillitoe, the ex-head of MI5, who was working in security for diamond-trading company De Beers. The meeting helped inspire the fourth James Bond Novel, Diamonds Are Forever. The book title came from a 1940s advertising campaign that over 30 years would launch an entire industry and establish “De Beers” as synonymous with diamonds. In the movie adaption starring Sean Connery, James Bond tricks the villains by swapping real diamonds for fake.
Whether it’s a thrilling escape, the heist of a century, or the secret handoff of a small velvet bag with considerable heft, humanity has been enthralled and fascinated by diamonds and their legitimacy. We see it all the time in the movies: characters attempting to ascertain if their ill-gotten diamonds are legitimate, or just so much useless glass.
Ways to Spot a Fake
In real life, there are several well-practiced methods for testing a diamond’s authenticity. For one, drop it in a glass of water. If it doesn’t sink directly to the bottom, it’s definitely not a diamond. Place a diamond under an ultraviolet light and note the reaction. If it emits a blue glow, it’s likely a diamond. If you place it on a piece of newspaper and can read the letters through it, it’s probably not a diamond (real diamonds refract the light so that you wouldn’t see the letters).
If you are able to take the diamond to a jeweler, they will likely use a loupe (that fancy eye piece) to look for small imperfections (called inclusions) that signify a real diamond. As diamonds are effective heat conductors, using a thermal conductivity probe can determine if it’s real. Use the device to heat the diamond and then measure how well it disperses the heat. If the heat disperses at a low rate, it’s not a diamond.
The best trained eyes can determine the veracity of a large carat diamond on sight. It will have that tell-tale sparkle, the blue-ish hue, a certain aura of magnificence. Synthetic diamond substitutes, called “simulants” in the industry, have tells that are easily picked out. A cubic zirconia stone does not refract light in the same way as a real diamond and lacks the sparkle of white light and will have an orange tint. If the stone seems blurred, it’s likely a white sapphire instead of a diamond.

Most diamonds aren’t big enough that their veracity can be noticed at a glance. Consider a diamond crusted band, for instance. Many tiny diamonds (less than 0.01 of a carat) are set on a piece of metal. What if some of those diamonds are actually cubic zirconia? Most people would never be able to tell, and even many retail jewelers wouldn’t notice. Or when a jeweler receives an order of hundreds or thousands of tiny stones. It’s extremely cumbersome to run every individual stone through tests to determine if they are legitimate.
De Beers helped pioneer a new, inexpensive method for testing these small stones, using near infrared and time-gated imaging to tell colorless cubic zirconia from the real deal.
The Existential Threat of Cubic Zirconia
According to a research paper published in Minerals by diamond company De Beers and the Imperial College of London in September 2020, cubic zirconia is “an excellent simulant because of high brilliance, hardness, chemical inertness, low dispersion, isotropic crystal structure, high transparency and flawlessness and its high band gap, hence near-colorless appearance.”
Cubic zirconia is easy and inexpensive to make and the diamond industry has long considered it an existential threat. Not many people are buying cubic zirconia instead of diamonds, but its presence makes people often wonder about the veracity of their own diamonds. Even with decades of market awareness and a variety of methods available to detect cubic zirconia, people still try to pass it off as real diamond.
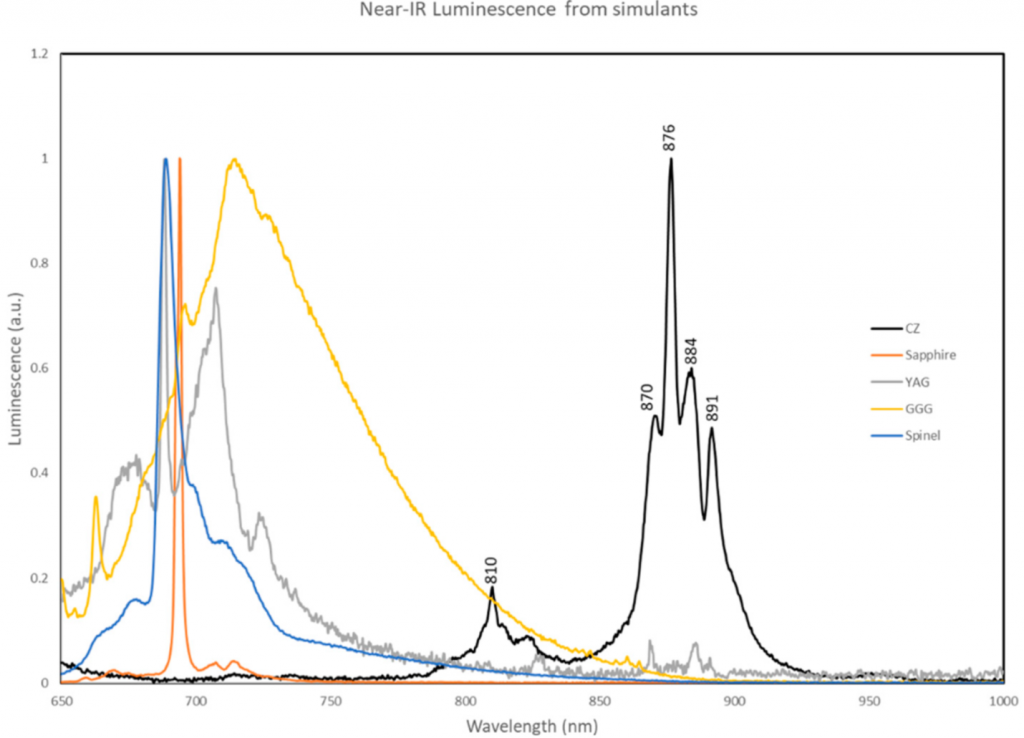
And yet, cubic zirconia stones are easy to spot based on certain properties imbued during the skull melting manufacturing process. For instance, using near infrared to measure the luminescence of various stones, cubic zirconia will have a distinctive spike between the 850 and 900 nanometer wavelengths, in comparison to diamonds or other simulants.
Testing Cubic Zirconia With Near-Infrared Luminescence
Luminescence is the emission of light by a substance that has not been heated. Fluorescence and phosphorescence are the two primary types of luminescence, characterized by how long light lingers in the object before dissipating. If the light disappears quickly, it is fluorescence. If light lingers in the object (for seconds or even hours), it is phosphorescence. Luminescence is often tested by “exciting” the electrons of an object by shining light on them and measuring the emission along a wavelength spectrum. Different kinds of chemicals have different wavelength signatures, thus allowing scientists to study the composition of objects near and far, such as the atmosphere of exoplanets, or the chemical composition of Renaissance paintings.
To help solve the issue of detecting cubic zirconia in tiny stones, the researchers from De Beers and the Imperial College of London tested over 1,000 commercially available cubic zirconia stones ranging in size from one to five millimeters from manufacturers such as Swarovski, Pandora, Ceres Crystal US and Messi Gems.
Researchers used two different methods to collect data:
- Sub-millisecond time-gated imaging of near-infrared luminescence was carried out using Teledyne DALSA’s Genie Nano M2050 monochrome CMOS area scan camera’s advanced cycling features.
- Spectral data was collected with an Andor iStar DH320T-18U-E3 intensified CCD camera via a Horiba iHR-320 spectrometer.
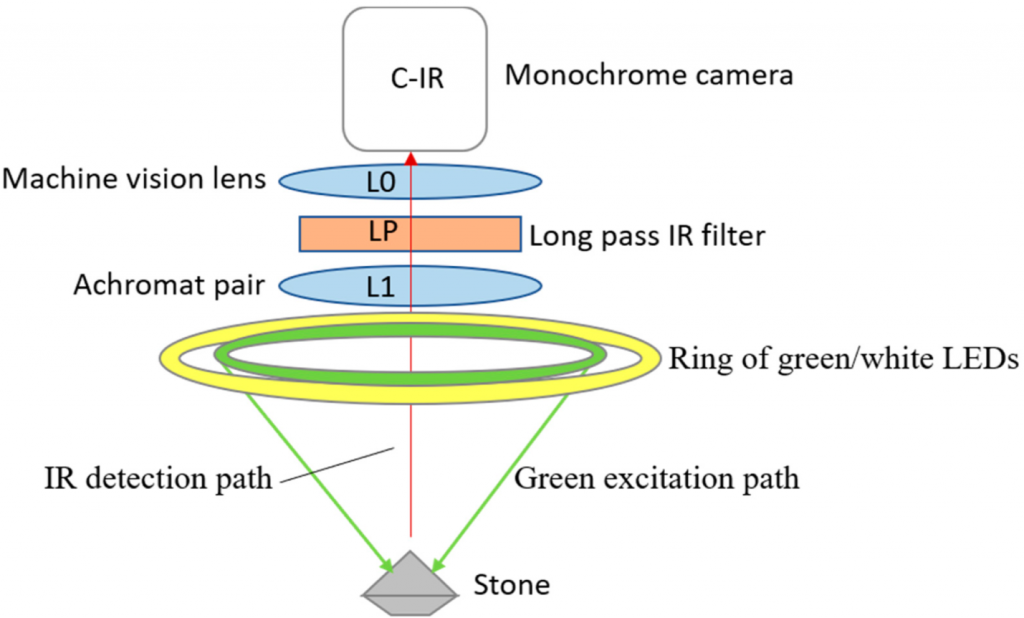
For the time-gated imaging, a stone was set on a surface and then excited with white and green LED lights. The light was reflected through an infrared filter and a machine vision lens to the monochrome camera. The equipment was relatively inexpensive, with a total cost of about 1,000 euros in parts.
Because of the way that cubic zirconia is made, specific chemicals can be detected using near infrared luminescence. One common method to make colorless cubic zirconia uses yttrium oxide, which results in a specific kind of variation called yttrium-stabilized zirconia (YSZ). The spectrum can also show trace amounts of neodymium, a rare earth element, that is used to enhance or change the color of cubic zirconia.
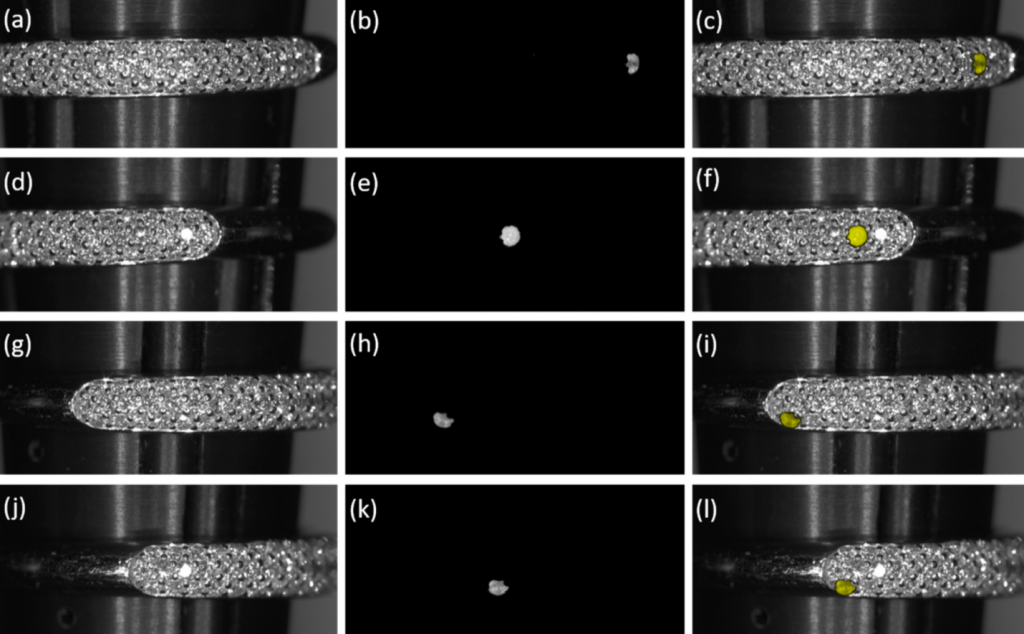
The use of near infrared, time-gated detection and spectral analysis was used on loose and banded diamonds and cubic zirconia. The method was able to discern the tiny cubic zirconia stone from a band crusted with otherwise viable diamonds, as well as among loose stones.
An Inexpensive Method to Test Tiny Stones
The primary methods for testing diamonds, either by using a loupe or a gemmological lamp to look for the blue hue luminescence of diamonds, are not conducive to analyzing large amounts of small stones.
“We believe one of the great advantages of this system is in its potential capability to rapidly screen and therefore differentiate [cubic zirconia] in jewelry containing very small diamonds,” researchers wrote.
Other methods require more time and more expensive equipment. Researchers were able to tackle the problem of identifying cubic zirconia in batches of small carat stones with about 1,000 euros worth of equipment and software.
Cubic zirconia has a delayed luminescence compared to real diamonds, leading researchers to note, “it is recommended that any stone exhibiting strong delayed luminescence in the near infrared be treated with caution, as this is not a typical feature found in this precious gemstone.”
Prioritizing solutions to match the needs of your industry


 Finding the Right Vein: Waterloo Students Propose Low-Cost NIR Imaging Solution for Venipuncture
Finding the Right Vein: Waterloo Students Propose Low-Cost NIR Imaging Solution for Venipuncture  Infrared-Based Imaging in Forensics
Infrared-Based Imaging in Forensics 