What Is Light Sheet Microscopy?
A Popular New Format For Microscopy
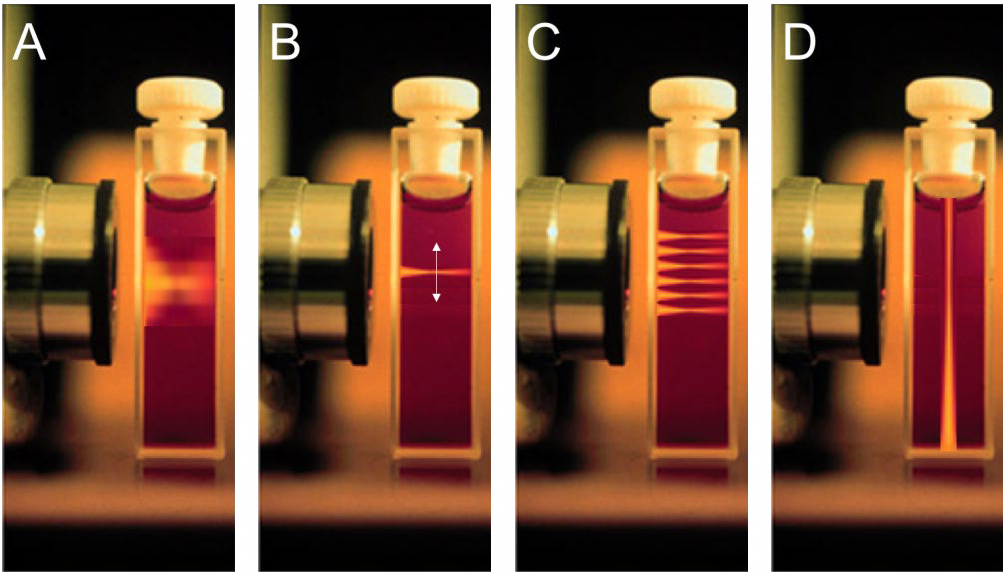
Conventional fluorescence microscopy involves flooding the whole sample with light and receiving emission light from the focal plane and also out-of-focus areas, resulting in lots of background fluorescence (Fig.1A). The signal level can be improved but involves using more intense laser light, which often results in phototoxic effects that can damage and eventually kill the sample organism, as well as bleaching any fluorescent markers in the sample. This combination of poor image quality, lack of reliable 3D imaging and gradual degradation of sample due to intense light means that alternatives to conventional widefield fluorescence microscopy are necessary.
One alternative is confocal microscopy, which uses pinholes to block all out-of-focus light and only detects light from the focal plane (Fig.1B, Fig.2A). The use of pinholes results in a higher contrast image with a much better signal to noise ratio. The pinholes also allow for optical sectioning, where images of different 2D focal planes can be built up into a 3D image without having to physically section the sample.
Spinning disk confocal microscopes allow for high-speed, high-quality 3D images of live biological samples, however, although pinholes stop out-of-focus light from affecting the resulting image, these out-of-focus areas are still exposed to light and emit fluorescence, it just is blocked by a pinhole and not detected, so the sample can still undergo photo-damage.
An ideal alternative fluorescence technique for 3D live cell imaging over long timescales would:
- Minimally photo-damage and bleach live samples so observations can last longer
- Observe fast 3D dynamic processes over long time periods
- Image large, living 3D specimens without having to mount/fix on a coverslip or flat surface
- Freely move the sample in 3D, including rotation
- Avoid out-of-focus fluorescence data to reduce out-of-focus light
- Allow for easy optical sectioning
This alternative is light sheet microscopy, popularized in 2004 as single plane illumination microscopy (SPIM). The differences between widefield, confocal and light sheet illumination methods can be seen in Figure 1.
Light Sheet Microscopy
In typical microscopy techniques, the illumination and imaging systems use the same light path and are on the same axis. In light sheet microscopy, the illumination beam is perpendicular to the imaging system (Fig.1D, Fig.2B), creating a sheet of light through a particular 2D plane of the sample. This imaging plane is the same as the focal plane, and there is little excitation above or below, meaning there is minimal out-of-focus light.
By using a lens shaped like a cylinder, excitation light is shaped into an hourglass as the light is compressed in one dimension but not the other (Fig.2C-D). This shape allows for a sample to be located in the thin ‘waist’ of the beam (where the sheet is focused) where only a small plane of the sample is excited. The emitted fluorescence is detected up by a different objective which leads to the camera.
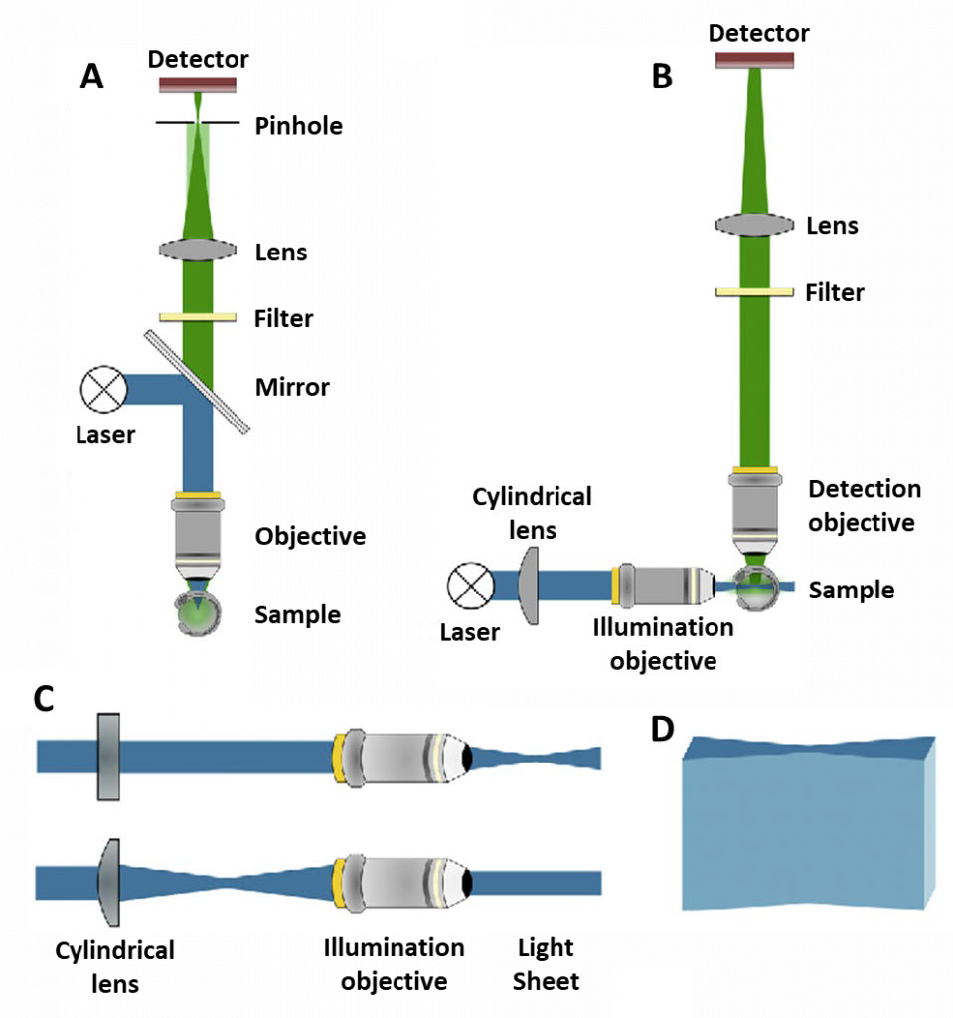
This imaging setup has multiple benefits. Firstly, a single 2D plane of the sample can be imaged quickly, meaning fast dynamic processes can be seen. Secondly, only one plane is being excited, meaning that there is far less out-of-focus light (when compared to widefield), which improves signal.
In many light sheet systems, the optics are static and won’t move, meaning that the sample needs to be moved if the image needs to be adjusted. This means the sample is prepared on a moveable stage which can move in the three main dimensions x, y and z, as well as rotational movement. Movement in xyz allows the light sheet to scan across a large sample, with the images stitched together digitally to create an image larger than the field of view of the camera. Rotational movement allows viewing of the sample from multiple angles and a 3D image can be produced, which is higher quality due to multiple angle images being fused together.
Scanning over samples in xyz/rotation in light sheet microscopy allows for a high-quality composite image to be built within seconds to minutes, as opposed to minutes to hours when using a confocal microscope. In addition, even when moving the sample through the light sheet the exposure and photo-damage is reduced significantly, which allows for imaging live for much longer and obtaining more relevant data.
Light Sheet Implementations
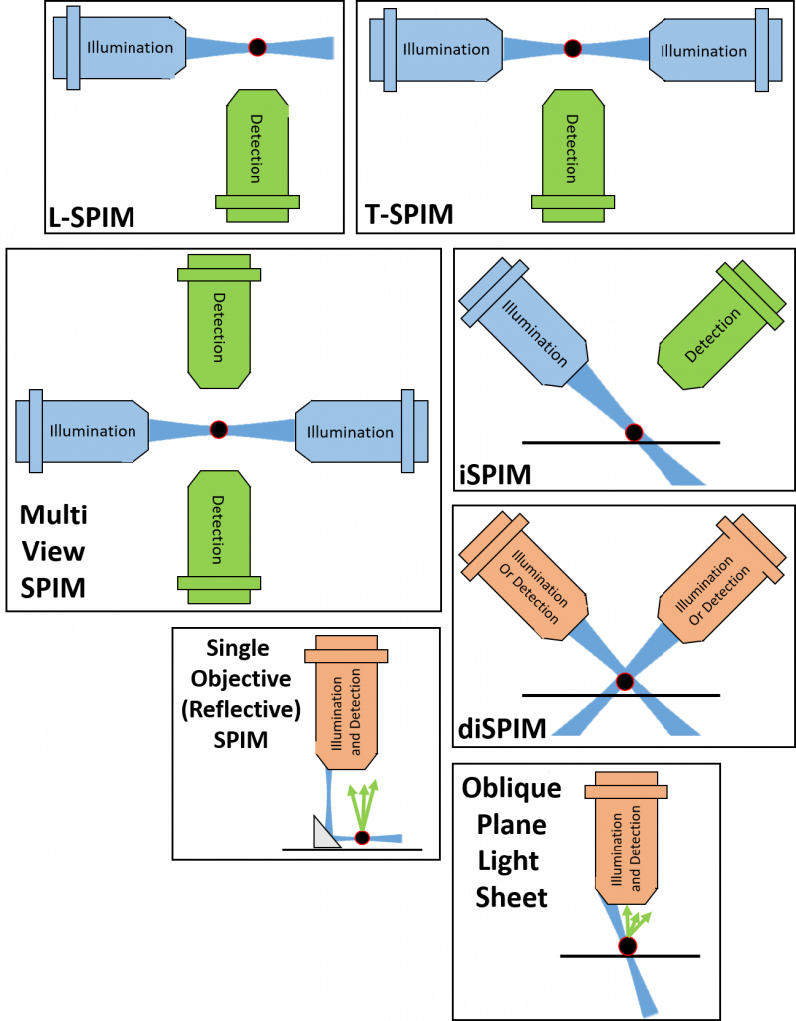
There are many different types of light sheet fluorescence microscopy, mostly based on the original SPIM design, making changes depending on the sample, application, and scientific question to answer.
Light sheet microscopy is a very active area of development and new configurations are created all the time. While the initial SPIM (and close SPIM variants) use one objective for illumination and another for detection, newer light sheet methods are known as ‘single-objective’ light sheet, using one objective for both illumination and detection, bypassing previous limits on the type of objective and restrictive sample geometry. Figure 3 shows a summary of some light sheet variants.
Other advances in light sheet involve changing the nature of the light sheet itself, such as using a scanning beam for axially scanned light sheet (ASLM) and virtual light sheet, or by using a Bessel beam for super-resolution light sheet.
Light sheet microscopy is a rapidly evolving but also open-source area, with resources such as OpenSPIM allowing researchers to easily build their own light sheet system with detailed guides and parts lists.
Cameras For Light Sheet
Due to light sheet having such a wide variety of implementations, it is vital to use a flexible camera for light sheet so that the camera can adapt to future system modifications. In general, light sheet aims to use a very low light dose in order not to disturb the sample, meaning a sensitive camera is paramount in order to detect low signal levels without interference from noise.
Other light sheet systems such as mesoSPIM involve looking at large samples in the centimeter scale, far larger than typical microscopy samples. This would require a camera with a large field of view and a small pixel size, in order to image large samples in as few images as possible, while retaining a high resolution.
In addition, light sheet can be paired with functional imaging techniques such as calcium or voltage imaging, requiring high speed imaging. A camera with fast readout times is necessary in order to not be a bottleneck for system speed and to capture fast, dynamic events.
Overall, a camera for light sheet really needs to do it all.
Summary
Light sheet is a rapidly growing field in imaging due to its flexibility, low cost, and power as a platform. With the low phototoxicity, ability to image large samples and acquisition of images at a high speed, light sheet is the technique of choice for a number of researchers. The many flavors of SPIM allow for a customizable platform to be built in-house, for any imaging needs.


 Spinning Disk Confocal Microscopy
Spinning Disk Confocal Microscopy  Super resolution microscopy
Super resolution microscopy 